Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Spectrophotometric Method Development for Estimation of Multicomponent Antidiabetic Drugs in Bulk and Solid Dosage Form
Authors: Aslam A. Sayyad, Sanjay B. Deshmukh, Ravi U. Kurhade
DOI Link: https://doi.org/10.22214/ijraset.2023.56021
Certificate: View Certificate
Abstract
The aim of the present study was the development and validation of a simple, precise and accurate UV-spectrophotometric method for the estimation of metformin (MET), voglibose (VOG) and pioglitazone (PIO) in bulk and tablet dosage form using methAanol as solvent. The method was proposed in the present work, the maximum absorbance was shown at 236nm for MET, 256nm for VOG and 268nm for PIO. The concentration range was 5-25, 0.2-1 and 2-10?g/mL with correlation coefficient 0.0998, 0.999 and 0.999 for MET, VOG and PIO respectively. The various parameters, such as linearity, system suitability, accuracy, precision, ruggedness, limit of detection and limit of quantification were studied as per ICH guidelines. Accuracy of the method was verified by performing recovery studies using simultaneous equation method and found to be 100.04-100.48% for MET, 98.70-99.70% for VOG and 98.67-100.27% for PIO indicates good accuracy of the method. Excellent mean recovery studies for precision, repeatability, ruggedness and sensitivity results showed that the method has been validated successfully, the results are also in accordance with the % RSD values obtained within specified limits. The proposed method was applied to the determination of MET, VOG and PIO, the mean % amount was found to be 100.21 (MET), 98.70 (VOG) & 101.28 (PIO) with % RSD values NMT 2.0% indicates the developed method was successfully applied for analysis of marketed formulation. The developed spectrophotometric method can be employed for routine analysis of MET, VOG and PIO in bulk and tablet formulation.
Introduction
I. INTRODUCTION
Ultraviolet visible spectrophotometry is most frequently employed techniques in pharmaceutical analysis. The photometric methods of estimations are based on the Bouger-Lambert-Beer’s law, which establishes the absorbance of a solution is directly proportional to analyte concentration and path length in the solution. It involves measurement of the amount of ultraviolet (190-380 nm) or visible (380-800 nm) radiation absorbed by a substance in a solution. Instrument, which measure the ratio, or a function of the ratio of the intensity of two beams of light in the ultraviolet visible regions are called Ultraviolet visible spectrophotometer [1]. A compound or drug posses a functional group, absorbs UV radiation at a specific wavelength and this character of the drug is specific for a fixed solvent system. The wavelength at which maximum absorption occurs is called as λmax. It is independent of concentration. With the help of Beer-Lambert’s law any molecule present as a single component system or multiple component system could be quantified effectively by UV spectroscopic method. For a drug to be measured by the ultraviolet analytical method it should follow the Beer- Lambert’s law [2], which is represented as

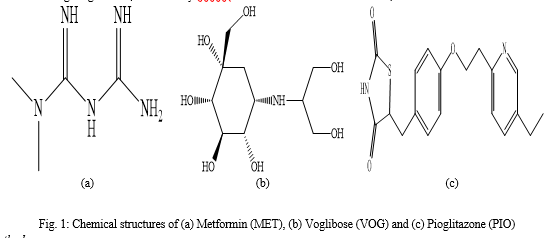
Where, A- absorbance, a-absorptivity, b- path length and c- concentration Metformin (MET) (Fig.1a) 3-(diaminomethylidene)-1, 1-dimethylguanidine is a biguanide hypoglycemic agent used in the treatment of non-insulin-dependent diabetes mellitus not responding to dietary modification. Metformin improves glycemic control by improving insulin sensitivity and decreasing intestinal absorption of glucose [3]. Voglibose (VOG) (Fig.1b) (1S,2S,3R,4S,5S)-5-(1,3-dihydroxypropan-2-ylamino)-1-(hydroxymethyl) cyclohexane-1,2,3,4-tetrol is a valiolamine derivative and inhibitor of α-glucosidase with antihyperglycemic activity. Voglibose binds to and inhibits α-glucosidase, an enteric enzyme found in the brush border of the small intestines that hydrolyzes oligosaccharides and disaccharides into glucose and other monosaccharides. This prevents the breakdown of larger carbohydrates into glucose and decreases the rise in postprandial blood glucose levels [4]. Pioglitazone (PIO) (Fig.1c) 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione is a thiazolidinedione and it is selective agonists for the nuclear peroxisome proliferator-activated γ-receptor (PPARγ) which enhances the transcription of several insulin responsive genes.
They tend to reverse insulin resistance by stimulating GLUT4 expression and translocation therefore entry of glucose into muscle and fat is improved. Hepatic gluconeogenesis is also suppressed. Activation of genes regulating fatty acid metabolism and lipogenesis in adipose tissue contributes to the insulin sensitizing action [5]. The literature survey reveals that various methods are present for the determination of metformin, voglibose and pioglitazone, individually or in combination of listed two drugs. The methods, UV-spectroscopy and HPLC are already developed in combination for metformin and voglibose and for metformin and pioglitazone. There are no any analytical method reported previously for the simultaneous estimation of voglibose, pioglitazone and metformin in multi component dosage form. Therefore, it is aimed to develop a simple, accurate, sensitive and reproducible method for combined voglibose, pioglitazone and metformin in bulk and tablet dosage form by UV-spectrophotometry.
II. MATERIALS AND METHODS
A. Materials
Metformin, Pioglitazone and Voglibose were received as gift sample from Macloids Pharmaceuticals Ltd. Gujrat, India. Methanol of HPLC grade was procured. The D-Bose MP275 tablet as a marketed formulation used which contains 0.2mg Voglibose, 500mg Metformin and 7.5mg Pioglitazone, marketed by Sinsan Pharmaceuticals PVT. LTD. Pune, India.
B. Methods
- Instrumentation
Spectrophotometric analysis was performed on a double beam UV/Visible spectrophotometer, Shimadzu (UV 1800), Software UV Probe V2.42. Ultrasonicator, Spectralab (UCB30) was used for sonication. Standard and sample drugs were weighed by using Contech (model 1473) digital analytical balance.
2. Selection of solvent
Solubility studies were done by dissolving drugs in solvents like water and methanol. It was observed that Metformin (MET) was freely soluble in water and methanol but Voglibose (VOG) and Pioglitazone (PIO) were sparingly soluble in water forms turbidity and freely soluble in methanol therefore methanol was selected as a common solvent.
3. Preparation of standard solution for MET, VOG and PIO
Standard stock solution of MET, VOG and PIO was prepared by dissolving 10mg of each drug separately in 100ml volumetric flask using methanol as solvent up to 100ml and each sample sonicate up to 15min. Stock solution of 100μg/mL were obtained. From these stock solutions, working stock solutions of concentration were prepared by appropriate dilutions.
4. Selection of wavelength
The working standard solutions of MET, VOG and PIO were scanned in the entire UV range of 400-200nm to determine λmax. The λmax of MET, VOG and PIO were found to be 236nm, 256nm and 268nm respectively.




V. DISCUSSION
An attempt was made to develop UV spectrophotometric method for the estimation of Metformin, Pioglitazone and Voglibose in bulk and tablet dosage form. Solubility studies were done by dissolving drugs in solvents like water and methanol. It was observed that Metformin (MET) was freely soluble in water and methanol but Voglibose (VOG) and Pioglitazone (PIO) were sparingly soluble in water forms turbidity and freely soluble in methanol therefore methanol was selected as a common solvent.
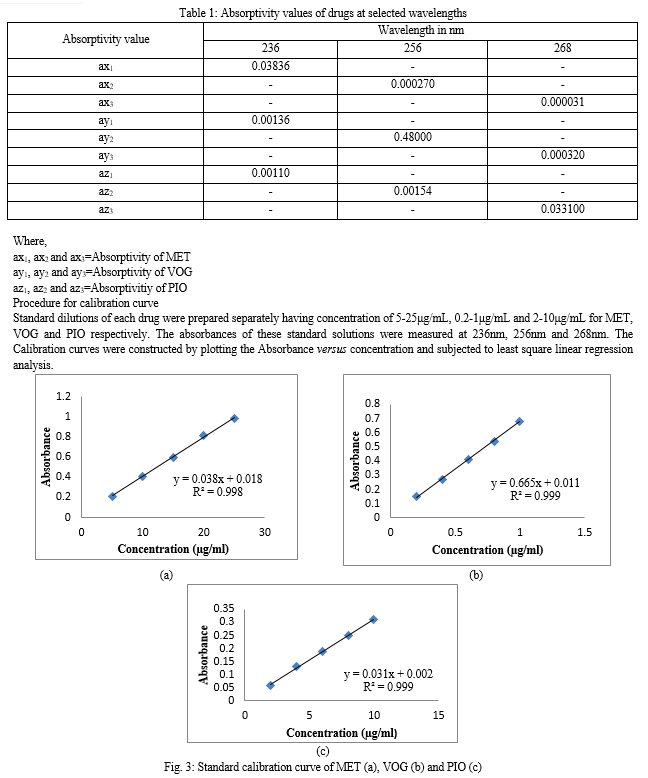
The working standard solutions of MET, VOG and PIO were scanned in the entire UV range of 400-200nm to get absorbance spectrum. UV- Visible spectra and overlay spectra of MET, VOG and PIO are shown in Fig.2. From the absorbance spectra, three wavelengths 236nm (λmax of MET), 256nm (λmax of VOG) and 268nm (λmax of PIO) were selected for estimation of these drugs using Simultaneous Equation Method (SEM). The % RSD found were within 2.0%, which indicates that the system is precise to analyze the sample. Accuracy is the closeness of the best result obtained by the method to the true value. The concentration recovered should be within ±2% to the true value.
Accuracy of the developed method was confirmed by recovery study as per ICH norms at three different concentration levels of 80 %, 100 %, and 120 %. Here to a preanalysed sample solution, standard drug solutions were added and then percentage drug content was calculated. Amount of the drug recovered was calculated using simultaneous equation method for accuracy. The percentage of the standard added to the pre analyzed sample was calculated and it was found to be 100.04-100.48% for MET, 98.70-99.70% for VOG and 98.67-100.27% for PIO indicates good accuracy of the method (Table 4.1, 4.2). The recovery study results with statistical validation have shown in Table 4.3. In determination of precision, the %RSD, were not more than 0.25%, 0.87% and 0.51% for MET, VOG and PIO respectively, indicating the method was precise and results are shown in Table 5. Repeatability was determined by the analyzing MET (25μg/mL), VOG (1μg/mL) and PIO (10μg/mL) of drug solution for three replicates and results are shown in Table 6.1, 6.2 and 6.3. The repeatability again shows the closeness of the observed results that enhance the reliability of the above method. LOD for MET, VOG and PIO were found to be 0.42, 0.02 and 0.25μg/mL respectively. LOQ for MET, VOG and PIO were found to be 1.27, 0.06 and 0.75μg/mL respectively. The mean standard deviation is 0.005, 0.004 and 0.002 and slope was 0.038, 0.665 and 0.031 for MET, VOG and PIO respectively. Ruggedness of the proposed method was studied by two different analysts using the same experimental and environmental conditions; the results are given in Table 7. The % RSD was found to be 0.32- 0.90% for MET, 0.38-0.46% for VOG and 0.34-0.76% for PIO respectively.
Conclusion
The developed spectrophotometric method was found linear over wider concentration range. Therefore the developed spectrophotometric method can be applied for routine quantitative and qualitative analysis of MET, VOG and PIO in bulk and pharmaceutical formulations. The proposed method based on the UV-spectrophotometry is suitable for determination of MET, VOG and PIO in tablet formulation. Method developed can be conveniently used for quality control and routine determination of drug in pharmaceutical dosage forms in pharmaceutical industry.
References
[1] Cannors KA. A textbook of pharmaceutical analysis. In: Absorption spectroscopy. 3rd ed. New York. John Wiley & Sons 1982. pp. 173-247. [2] Sharma B. K. Instrumental methods of chemical analysis. In: Spectroscopy. 24th ed. Meerut. Krishna prakashan media 2005.pp. 72-80, 114-119. [3] Pubchem [Homepage on Internet]. National Center for Biotechnology Information. PubChem Compound Database; CID=4091 [cited 2017 Aug 14]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/metformin. [4] Pubchem [Homepage on Internet]. National Center for Biotechnology Information. PubChem Compound Database; CID=444020 [cited 2017 Aug 14]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/voglibose. [5] Tripathi KD. Essentials of medical pharmacology. In: Insulin, oral hypoglycemic drugs and glucagon. 6th ed. New Delhi. Jaypee brother’s medical publisher’s ltd 2008. pp. 266-270. [6] ICH Topic Q2A. Validation of analytical procedures methodology. CGMP/ ICH/281/95.1995. [7] Mohamed MA, Mohamed Abd A, Mohamed A. Simultaneous determination of metformin and pioglitazone in pharmaceutical dosage form by HPLC method. International Journal of Pharmaceutical Sciences and Research 2014; 5 (6): 2569. [8] Kadam V, Yadav P, Mohite S, Magdum C. Development and validation of analytical methods for simultaneous estimation of voglibose, glimepiride and metformin hydrochloride in bulk and tablet dosage form by HPLC. International Journal of Pharmacy and Pharmaceutical Research 2014; 1 (2): 10-21. [9] Singh V, Chaudhary P, Tiwari R. Method development of Pioglitazone by UV Spectrophotometer. International Journal of Drug Development and Research 2014; 6 (4): 80-83. [10] Neelima K, Prasad Y. Analytical method development and validation of metformin, voglibose, glimepiride in bulk and combined tablet dosage form by gradient RP-HPLC. Pharmaceutical Methods 2014; 5 (1): 27-33. [11] Shaik S, Joshi K, Usha M, Bindhu T, Ramya T. Analytical method development and validation of pioglitazone hydrochloride by RPHPLC. J Chem Pharm Res. 2014; 6 (6): 16-21. [12] Sonia K., Prasad B. RP-HPLC analysis of metformin hydrochloride and voglibose and study of its different analytical parameter. International Journal of Pharmaceutical Sciences and Research 2013; 4 (6): 2252. [13] Todkar S, Mohite S, Mali S, Rananavare S. Development and validation of uv spectrophotometric methods for simultaneous estimation of voglibose and metformin hydrochloride in bulk and tablet dosage form. Indo American Journal of Pharmaceutical Research 2013; 3 (9): 7018-7024. [14] Tengli A, Gurupadayya B, Soni N, Vishwanathan B. Method development and validation of metformine, pioglitazone and glibenclamide in tablet dosage form by using RP-HPLC. Biochem Anal Biochem 2013; 2 (130): 2161-1009. [15] Pallavi P, Rathod S, Chaudhari P. Development and validation of UV derivative spectrophotometric methods for the determination of glimepiride, metformin HCl and pioglitazone HCl in bulk and marketed formulation. J. Pharm. Sci. Innov 2012; 1: 58-62. [16] Raj N, Bhatt M, Kabra P, Kimbahune R. Simultaneous quantification of voglibose and metformin by validated analytical method in tablet dosage form. International Journal of Pharmacology and Technology. 2011; 3 (2): 53-56. [17] Sujana K, Swathi R, Bhanu P, Reddy S. Simultaneous estimation of pioglitazone hydrochloride and metformin hydrochloride using UV spectroscopic method. J Biomed Sci Res. 2010; 2 (2): 110-115. [18] Saxena P, Raghuwanshi A, Jain U, Patel A, Gupta N. UV spectrophotometric method for the quatitation of metformin hyrochloride in pharmaceutical dosage form. Oriental journal of Chemistry 2010; 26 (4): 1553. [19] Lakshmi K, Rajesh T, Sharma S. Simultaneous determination of metformin and pioglitazone by reversed phase HPLC in pharmaceutical dosage forms. International Journal of Pharmacy and Pharmaceutical Sciences 2009; 1 (2): 162-166. [20] Jain D, Jain S. Amin M. Simultaneous estimation of metformin hydrochloride, pioglitazone hydrochloride, and glimepiride by RP-HPLC in tablet formulation. Journal of chromatographic science 2008; 46 (6): 501-504. [21] Pubchem [Homepage on Internet]. National Center for Biotechnology Information. PubChem Compound Database; CID=4829 [cited 2017 Aug 14]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Pioglitazone.
Copyright
Copyright © 2023 Aslam A. Sayyad, Sanjay B. Deshmukh, Ravi U. Kurhade. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET56021
Publish Date : 2023-10-05
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

